

Prior Authorization Requirements Continue to Impact Providers
August 08, 2024Authors
Topics
Welcome to the August 2024 edition of our Monthly Insight Series! This month we are combining insights from 3 of our customer segment reports and reflecting on provider and payer relationships.

Payers systematically implement prior authorization (PA) requirements to monitor quality care and outcomes and manage costs in oncology care. Payers may approve or deny PA requests from providers based on appropriate use assessments and other factors as determined by the payer. Common PA denial reasons include not meeting National Comprehensive Cancer Network (NCCN) standards and formulary exclusions or coverage policy. If a payer denies a PA, providers may choose to appeal or switch to the payer preferred treatment option. If a provider appeals, payers may either approve or deny these appeals. See Figure 1 a visual of the provider actions and payer response options.
PA requirements create additional steps and effort for providers and can be effective payer tools to influence provider behavior. Payers may effectively steer oncologists towards different brands in cases where oncologists believe that two treatments have equivalent efficacy and safety.
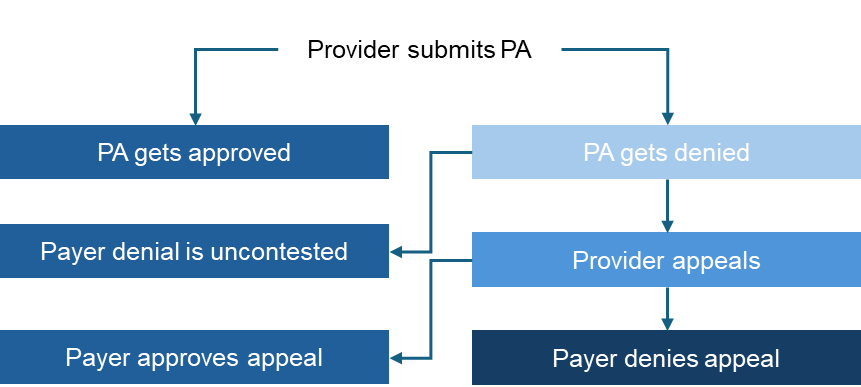
Figure 1. Provider Action/Payer Response Options
Source: HMP Market Access Insights 2024 IDN, Community, and Payer Oncology Trend Reports.
Abbreviation: PA, prior authorization.
Integrated delivery networks (IDNs), community practices, and payers have different perspectives on the frequency of each of these steps. These perspectives offer insights into the overall denial rates and the different dynamics between IDNs and payers, as well as community practices and payers. Figure 2 illustrates the different perspectives of IDNs, community practices, and payers on provider and payer relationships.
- Prior Authorization Denials: Providers and payers agree that payers deny approximately 20% to 24% PAs. IDNs perceive a slightly higher denial rate than community providers and payers. This higher perceived rate could be driven by more complex patients and treatment protocols.
- Provider Appeals: Community practices report being less likely to appeal prior authorization denials than IDNs. Community practices report appealing 34% of denials compared to IDNs appealing 41% of denials. The higher rate of appeals by IDNs could reflect greater perceived influence over payers as well as more robust infrastructure or staffing at IDNs to support providers during the appeal process.
- Prior Authorization Appeal Denials: Community practices also report the highest denial rate of prior authorization appeals at 59%. This is another indication that community practices perceive less influence over payers than IDNs.
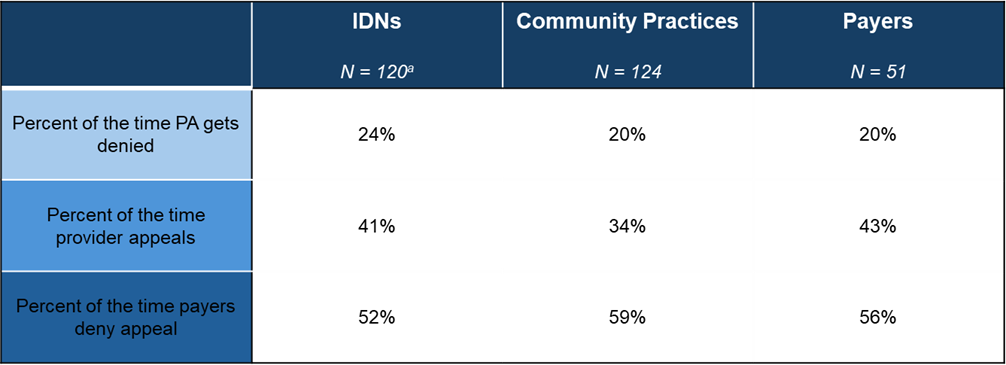
Figure 2. 2024 Estimated Average Level of Provider Action/Payer Response
aNote for IDNs N = 120 for PA denials, 122 for answered appeals, and 123 for answered appeals denied
Source: HMP Market Access Insights 2024 IDN, Community, and Payer Oncology Trend Reports.
Abbreviations: IDNs, integrated delivery networks; PA, prior authorization.
Additional insights can be found in our full HMP Market Access Insights’ reports!
Please reach out Ramon Auciello (rauciello@hmpglobal.com) with any questions you may have!
The Latest
Article
Thought Leadership Whitepaper: How Will Medicare Drug Price Negotiations Really Impact Providers?
As manufacturers prepare for Medicare drug price negotiations, a critical question emerges: How will your provider engagement strategy evolve when Maximum Fair Prices (MFP) take effect in 2026?
Emma BijesseArticle
Meet Dan: Researcher, Dad, and Oncology Report Innovator
At HMP Market Access Insights, we’re lucky to have a team of experts dedicated to uncovering meaningful insights in the oncology space. One of those experts is Dan, whose work is shaping how we approach community oncology research.
Daniel BuchenbergerArticle
Leveraging the IDN Dataset: Your Essential Tool for 2025
As IDNs face increasing complexity in oncology management, having a strategic approach backed by actionable insights is critical. Our dataset doesn’t just offer data—it equips your teams with the tools to anticipate challenges and seize opportunities.
Emma Bijesse





