

Article
IDNs’ Numerous Strategies and Tools to Manage High-Cost Drugs
May 13, 2024Authors
Topics
Welcome to the May 2024 edition of our Monthly Insight Series. This month we’re exploring the new tools IDNs have deployed to monitor high-cost drugs. This is a preview of findings from our 2024 IDN Oncology Trend Survey, coming at the end of May.

As the growth of drug expenses continues to outpace other expenses, integrated delivery networks (IDNs) are increasingly deploying sophisticated tools to monitor, manage, and influence prescribing behaviors. Our most recent 2024 IDN Oncology Trend Survey—scheduled for release at the end of this month—specifically looked at the use of high-cost drug review or committees because the majority of IDNs (~70%) use one of these mechanisms to consider both inpatient and outpatient treatments, which includes cancer drugs.
The Figure below illustrates the types and prevalence of tools IDNs with high-cost drug reviews or committees use to monitor these drugs.
- Of the IDNs with high-cost drug reviews, over 90% deploy two or more tools to monitor and manage these drugs.
- The most common tool was high-cost drug utilization reports, deployed by 84% of IDNs with high-cost drug reviews.
- In addition to retrospective reports, most IDNs with high-cost drug reviews have also implemented real-time alerts or tools to manage or guide prescribing. These include electronic health record alerts notifying providers of the need for additional approvals (75%), as well as real-time drug pricing tools (58%).
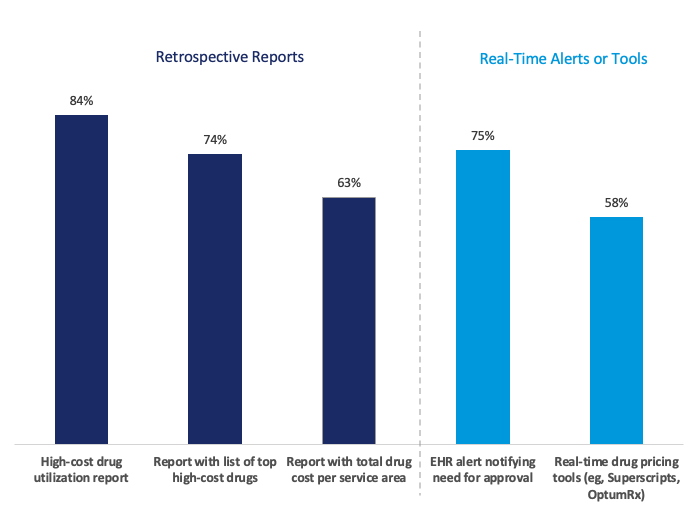
Figure. Share of IDNs With High-Cost Drug Reviews That Deploy Various Tools (2024; N = 112) Includes only IDNs that have high-cost drug reviews (69% of IDNs), and 1% of respondents were unsure.
Source: HMP Market Access Insights 2024 IDN Oncology Trend Report.
Abbreviations: EHR, electronic health record. IDN, integrated delivery network.
Manufacturers should be aware of how the variety of tools IDNs deploy to manage prescribing may influence prescribing patterns, and we look forward to sharing additional insights later this month when we release our full 2024 IDN Oncology Trend report!
The Latest
Article
Thought Leadership Whitepaper: How Will Medicare Drug Price Negotiations Really Impact Providers?
As manufacturers prepare for Medicare drug price negotiations, a critical question emerges: How will your provider engagement strategy evolve when Maximum Fair Prices (MFP) take effect in 2026?
Emma BijesseArticle
Meet Dan: Researcher, Dad, and Oncology Report Innovator
At HMP Market Access Insights, we’re lucky to have a team of experts dedicated to uncovering meaningful insights in the oncology space. One of those experts is Dan, whose work is shaping how we approach community oncology research.
Daniel BuchenbergerArticle
Leveraging the IDN Dataset: Your Essential Tool for 2025
As IDNs face increasing complexity in oncology management, having a strategic approach backed by actionable insights is critical. Our dataset doesn’t just offer data—it equips your teams with the tools to anticipate challenges and seize opportunities.
Emma Bijesse





